It's commonly assumed that synapses using GABA and glycine as neurotransmitters are inhibitory, that is when an action potential causes the release of (one or both of) these neurotransmitters (from the pre-synaptic side of the synapse) the result is a reduced chance that the post-synaptic cell will fire an action potential. However, this is not always true, there are several common circumstances under which these neurotransmitters appear to increase the chance of an action potential firing in the post-synaptic cell, as discussed in two recent papers to be discussed here. First, let's look at the mechanisms responsible for this reversal of the standard expectations, then I'll go over the two papers, then integrate the subject with some of my previous discussions regarding the transmission of analog data (besides action potential timing) along the first few hundred microns of the axon.
The role of Chloride Currents in Neural Communication
In a recent post I described the general relationship of various currents, ion channels, and voltage events (e.g. spikes and action potentials) in the neural cell membrane, but deferred discussion of chloride (Cl-) currents because of their complexity.
The key item regarding any ion-carried current is the equilibrium voltage (also often called the reversal potential): that voltage (across the cell membrane) at which the electrical pressure driving a specific ion in one direction exactly balances the concentration-driven pressure in the other. The equilibrium voltage is primarily determined by the relative concentrations on either side of the cell membrane, so a change to either concentration can produce a change to the equilibrium voltage, and different concentrations across different parts of the cell (membrane) will result in different equilibrium voltages. (They will also result in a net ion diffusion, along with an energy cost to maintain it: see below.)

Figure 1: Action Potential Threshold and Directions of Depolarization and Hyperpolarization Relative to Figure 3 of A New Integrative Theory for Cortical Pyramidal Neurons. (From Figure 4 of the same post.)
As you can see from figure 1, the typical equilibrium voltage for Cl- falls right into the range of typical "resting" voltages across the membrane. All of these are within about 40 mV of a typical action potential threshold, which is primarily determined by the nature of the specific voltage-gated Sodium (Na+) channels present in that piece of membrane. (See my discussion here for more detail.) Now, let's compare the effects of changing the Cl- concentration inside the cell from that of the soma (body) of a typical pyramidal cell to that of the axon initial segment[A1] (AIS: the first part of the axon after it leaves the axon hillock).

Figure 2: Effect of Cl- current with Equilibrium voltage at ~-73mV as typical for a pyramidal cell soma.[1] (Original. You may link to, copy, and or modify this image.)
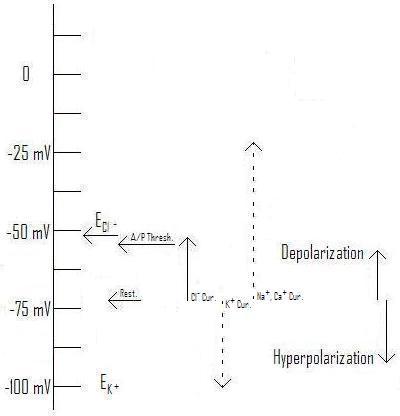
Figure 3: Effect of Cl- current with Equilibrium voltage at ~-54mV as typical for a pyramidal cell AIS.[1] (Original. You may link to, copy, and or modify this image.)
As you can see, raising the equilibrium voltage for Cl- changes the direction of the current and its effect. Rather than being inhibitory (hyperpolarizing), the chloride current is excitatory (depolarizing), tending to make the post-synaptic cell more likely to fire an action potential. Notice in this illustration (Figure 3) the equilibrium voltage is higher than the threshold for firing an action potential, so enough chloride current can "do the job" independently of help from depolarizing influences coming from the dendrites. This has actually been observed in experiments.[1] [21]
As mentioned in A New Integrative Theory for Cortical Pyramidal Neurons, the normal concentration difference is just about enough to create an equilibrium voltage in the same range as the typical resting potential. This is because the concentration of Cl- outside the cell is greater than that inside by just enough to counteract the resting voltage. If, somehow, a higher concentration of Cl- is present on the inside of the membrane, the equilibrium voltage will rise, as shown in Figure 3 for the AIS.
Now, we can't just leave it that the inhibitory effects of neurotransmitters such as GABA and glycine can be reversed, we need to consider both the receptors (all types) and and the effects of changing the voltage under inhibitory conditions. ... (read the rest in the full post)
Let's start with Figure 2, when a big dose of GABA arrives at a synapse. The fastest-acting receptor(s) for GABA are the GABAA receptors, in which the molecule that spans the membrane contains both a pore through which the Cl- ions can pass, and a receptor region (outside the cell) that fits to GABA like a lock to a key, changing its shape (and the shape of the molecule), and allowing the pore to open. (A good discussion of this receptor may be found at The Versatile GABAa Chloride Channel Receptor Complex at Physiology physics woven fine. I'm not going to try duplicate this work, but in reading it just keep in mind that the author has not considered that the GABAA receptor can sometimes be excitatory.)
Notice, though, that the difference in voltages is very small. This isn't as important is it might seem, because when the depolarizing activity of an excitatory synapse (a regular one using i.e. glutamate or acetylcholine) tries to raise (depolarize) the voltage towards the threshold, the greater difference in voltage drives a greater current, tending to prevent the voltage from going very far from the resting potential.
By contrast, a potassium (K+) current will pull the voltage much farther down, distinctly hyperpolarizing the cell membrane, not just at the synapse but a considerable distance away. This will become important later (below), because another GABA receptor, the GABAB receptor, can cause K+ channels to open, having just this effect. Individual cells can express different mixes of receptors, and even the same cell can have different mixes in different parts of the cell.[27] [28]
This isn't just true of receptors, either. Ion pumps can be localized, with massively different densities in different parts of the cell membrane (as can many other membrane proteins.[27]
Now, let's look at the AIS, in Figure 3. Here, a big dose of GABA opens the pores in the GABAA receptor in the same way as above, but it produces a distinct depolarizing current, pulling the membrane voltage, as always, towards its equilibrium voltage, which in this case actually happens to be higher than the action potential threshold. Thus, in this case the GABA can not only be excitatory, it can trigger an action potential. (Of course, if the post-synaptic cell also expresses GABAB receptors, the effect will be much more complex: see below.)
Chloride Concentrations and Their Maintenance
What are the typical CL- concentrations driving these different currents? I'm not going to go into the extra-cellular (concentration outside the cell) concentration, since they're the same for all parts of the cell. (Since different parts of different cells are bathed in the same local extra-cellular fluids.) Instead we'll look at the intra-cellular (concentration inside the cell) found by Khirug, Yamada et al., one of the papers we'll be discussing.[20]
By determining the reversal potentials mediated by sudden release of GABA("EGABA values of –59.4 ± 1.5 mV (n = 14), –65.8 ± 1.2 mV (n = 14), and –70.9 ± 1.5 mV (n = 10), respectively (Fig. 1)"[20]), they could calculate the concentrations:
Assuming an intracellular pH of 7.2, the intracellular levels of Cl– ([Cli]) calculated on the basis of the above EGABA values ([refs]) are 11, 7.9, and 6.0 mM [milliMoles, a measure of concentration] for the AIS, soma, and dendrite, respectively.[20]Of course these values are typical of only one type of cell (dentate gyrus cells (DGCs) of the hippocampus of mice and rats), but they give us some numbers to work with.
How are these concentrations maintained against the inevitable leaks and currents, which would normally push ECl- towards the resting voltage?[20] [33] There are two specific transporters involved (out of a large number used throughout the body): Both are members of a large family called cation–chloride co-transporters (CCCs), transmembrane proteins that transport a specific mix of ions in a compulsory group (all together or none). One is a potassium-chloride co-transporter (KCC): KCC2, while the other is a sodium-potassium-chloride co-transporter (NKCC): NKCC1. (The numbers are confusing, the "1" has nothing to do with the "2": the body also has KCC1 and NKCC2, although they aren't relevant to this discussion.)
KCCs, including KCC2, transport one K+ and one Cl- together, which is plenty to maintain the type of potential seen in Figure 2. The K+ has a strong pressure "pushing" it out of the cell, and it will drag the Cl- along with it. (Remember that since these ions have opposite charges, they represent currents going in opposite directions.)
NKCCs, including NKCC1, move one Na+, one K+ and two Cl- together, which means they will go in the other direction because the very high pressure "pulling" Na+ into the cell will trump the pressure of the other three ions.
During development, most neurons express NKCC1, making GABAA currents excitatory, which appears to be necessary for proper neural development.[2] [20] Mature neurons appear to mostly express KCC2 in the soma and dendrites, which makes Cl- currents hyperpolarizing. There has been some controversy over how the higher concentrations in the AIS were maintained, with the standard explanation being the absence of KCC2 in the AIS.
GABAergic Depolarization of the Axon Initial Segment in Cortical Principal Neurons Is Caused by the Na–K–2Cl Cotransporter NKCC1
This paper, (by Stanislav Khirug, Junko Yamada, Ramil Afzalov, Juha Voipio, Leonard Khiroug, and Kai Kaila,) investigates the conflict between the point (noted above) that the inevitable leaks and currents would normally push ECl- towards the resting voltage, and the fact that Cl- currents in the AIS have been observed to be distinctly depolarizing, which means that the concentration must be maintained at a level such that ECl- is distinctly higher than the resting voltage. A simple absence of KCC2, which would push ECl- lower, wouldn't cut it.
First, by subjecting different parts of the same cell to bursts of GABA (with any GABAB receptors blocked using "CGP55845A [(2S)-3-[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)-(phenylmethyl)phosphinic acid]"[20]) and measuring the resulting equilibrium voltages, they demonstrated the different values of EGABA for the AIS, soma, and dendrites. This may reasonably be assumed to be close to the value for ECl-, with only the GABAA receptors working.
Then, they did identical experiments similar to the one described above with both wild type mice (WT) and mutants unable to express NKCC1 anywhere(NKCC1–/–). The results:
The WT neurons showed an axo-somatic ΔEGABA that was similar (5 mV) to the one described above in the Thy1–mGFP neurons with AIS, somatic, and dendritic EGABA values of –61.3 ± 2.4 mV (n = 14), –66.0 ± 1.6 mV (n = 22), and –71.0 ± 2.0 mV (n = 16), respectively. Notably, in identical recordings from the NKCC1–/– neurons, there was no axo-somatic EGABA gradient; the values for EGABA at the AIS and soma were similar, –70.5 ± 1.5 mV (n = 17) and –70.3 ± 1.5 mV (n = 18), respectively. However, a somato-dendritic ΔEGABA similar to that in the WT neurons was still observed (dendritic EGABA of –74.6 ± 1.7 mV; n = 15). These data indicate a key role for NKCC1 in the generation and maintenance of the chloride gradient that results in depolarizing GABA responses in the AIS of mouse DGCs and suggest that NKCC1 has no significant influence on dendritic EGABA. Interestingly, the above observations are not compatible with the idea that the somato-dendritic EGABA gradient is set by NKCC1 and KCC2. The putative transport mechanism underlying this gradient is not in the focus of the present work, but preliminary observations point to a role of a bicarbonate-dependent somatic transporter that accumulates chloride in the DGCs (our unpublished data). [20]They have thus demonstrated, for two types of cells, the DGCs and "rat neocortical layer 2/3 pyramidal neurons", that the excitatory (depolarizing) action of GABA synapses onto the AIS is probably caused by the expression and localized targeting of NKCC1. They've also discovered another effect evidently not caused by NKCC1: the difference in Cl- concentrations between the soma and dendrites. It'll be interesting to see what further research turns up in this direction.
Complex Events Initiated by Individual Spikes in the Human Cerebral Cortex
This paper (by Gábor Molnár, Szabolcs Oláh, Gergely Komlósi, Miklós Füle, János Szabadics, Csaba Varga, Pál Barzó, Gábor Tamás) reports intriguing results from "complex events triggered by individual action potentials in the human neocortical network." Unlike previous research, they used slices of human brain with part of the small-scale network intact, and applied single strong spikes to a single cell and observed the result.
Before I go on with this, let me save a lot of readers the effort of digging into the paper over ethical concerns with this blockquote:
All procedures were performed according to the Declaration of Helsinki with the approval of the University of Szeged Ethical Committee. Human slices were derived from material that had to be removed to gain access for the surgical treatment of deep-brain tumors from the left and right frontal, temporal, and parietal regions with written informed consent of the patients (aged 18–73 y) prior to surgery over the last 4 y.[21](Anybody wanting more information can use part of the above blockquote in a text search in the article, which is open access.)
I'm not going to spend further on the methods, or the details of the results, instead jumping to a more "high-level" discussion, starting with another blockquote:
Our results show that a single spike of a pyramidal cell in the human cortical microcircuit is capable of activating complex sequences of postsynaptic potentials lasting an order of magnitude longer than detected previously [refs]. The initiation and internally precise temporal structure of these event series appears to follow a stereotyped mechanism of spike-to-spike transmission traveling through a subset of synaptically connected neurons that seems to be conserved across several brain regions of the human cerebral cortex. The flow of downstream activation that follows the first-order spike of the trigger pyramidal cell is directionally controlled at two consecutive synaptic steps. Second-order spikes are triggered exclusively in GABAergic interneurons and not in pyramidal cells due to interneuron-selective EPSPs of enormous amplitude. In turn, second-order spikes in axo-axonic cells give rise to third-order spikes detected only in pyramidal cells, resulting in trisynaptic EPSPs in the network because axo-axonic cells do not innervate other GABAergic cells [refs]. Synchronized to the spikes in axo-axonic cells, second-order spikes in basket cells and possibly in other types of interneuron [refs] elicit hyperpolarizing effects reported here as disynaptic IPSPs.What does this mean? The human brain, (or at least the parts of the neocortex investigated,) is a very complex network of cells with positive (excitatory) and negative (inhibitory) effects, in which a single spike applied to a resting network ends up producing a stereotyped mix of these effects. Basically, there is a "cascade" (a bad analogy, but...) of action potentials, starting with the artificially triggered
- "first-order spike", followed by one or more
- "Second-order spikes" in "GABAergic interneurons", including axo-axonic cells which have excitatory GABAergic synapses on the AISs of pyramidal cells, as well as "basket cells and possibly in other types of interneuron" which have inhibitory GABAergic synapses on the somas and dendrites of pyramidal cells, followed in turn by
- "third-order spikes detected only in pyramidal cells, resulting in trisynaptic EPSPs [excitatory postsynaptic potentials] in the network",
One of the key points, for our purposes, is that the "axo-axonic cells do not innervate other GABAergic cells", they terminate only on the AIS of pyramidal cells.[34] This means that the very excitatory GABAergic synapses we've been discussing play an essential role in this network process.
Beyond Action Potentials
So far, we've covered the currents resulting from action potentials in GABAergic synapses terminating on the AIS. But regular readers here will probably recall The Analog Axon, where I concluded:
It's not a totally new discovery, of course, that some nerve cells use more analog types of communication than just action potentials.[refs] Sensory nerves especially, often make use of analog calculations. Nevertheless, except for the sensory margins of cognition, the central nervous system is usually pictured as communicating through action potentials that carry only their relative timing as information. Over long distances, this is probably true.This is particularly important for the pyrmidal cells involved in the "third-order spikes" mentioned above, where the axo-axonic input has set up varying chloride currents--varying both along the AIS and over time. These currents set the membrane voltage, often to a point substantially above (more depolarizing) the nominal "resting" voltage, with equally varying effects on the size, length, and shape of the action potential, in addition to their contribution to the probability and timing of firing the action potential in the first place. While over longer distances these variations will go away, short collaterals to nearby neurons will cary them. Studies on the fixing the membrane voltage at the soma have shown effects propagating over 400 microns (μ),[15], and we can probably add the length of the AIS to similar effects generated by voltage changes there. Similar distances for propagating analog effects have been found in presynaptic hippocampal mossy fiber boutons,[14] the output synapses from mossy fiber cells where:However, a great many of the connections in the brain, including the neocortex, arguably the most important part of the relative expansion of the human brain, are within a few hundred microns, the distance discussed here.
Excitatory presynaptic potentials result from subthreshold dendritic synaptic inputs, which propagate several hundreds of micrometers along the axon and modulate action potential–evoked transmitter release at the mossy fiber–CA3 synapse.[14]Note that these effects in the dendrites have to cross the soma before they can extend "several hundreds of micrometers along the axon", presumably similar subthreshold synaptic inputs landing on the AIS would propagate farther.
How many synapses are we talking about to be affected by these inputs? From the research mentioned above fixing the membrane voltage of the soma:[15]
Layer 5 pyramidal neurons, like other cortical neurons, give rise to a local high density of axonal connections to other pyramidal and non-pyramidal cells[refs] (Supplementary Figs 1, 4 and 7). Indeed, examination of the main axon and local axon collaterals of biocytin-filled layer 5 pyramidal cells from our slices revealed, on average, 155 (±79; n=14 cells) putative synaptic boutons within 0.5-mm of the cell body, and 269 (±152) putative synaptic boutons within the first 1mm(Fig. 4c and Supplementary Fig. 7). These values are probably a significant underestimate of local synaptic connectivity, owing to the cutting of axons and limitations of axonal staining using the slice technique (see Supplementary Figs 1 and 4).[15]This means that the AIS and short collaterals of each pyramidal cell can potentially act as an independent integrator of axo-axonic inputs, feeding a real-time analog output from hundreds of inputs[31] to hundreds of outputs[15] through the size, duration, and shape of action potentials, as well as sub-threshold changes that could affect the size of the transmitter release burst at the synapse. Moreover, these effects are tunable: addition of GABAB receptors to a synapse could produce a longer-term (50-300 ms) K+ current counteracting the depolarizing effect when many bursts of GABA arrive within a short time.
Like many other recent discoveries, these add yet more complexity, and potential calculating power, to the overall activity of the brain. This is an effect that will be very difficult to model in the more traditional neural networks, hammering home yet again how we must not limit our ideas of the brain's ability by what current models and neural networks can do. There's more where that came from, and the next decade will almost certainly see increasingly complex and powerful models of what the brain can do.
Khirug, S., Yamada, J., Afzalov, R., Voipio, J., Khiroug, L., & Kaila, K. (2008). GABAergic Depolarization of the Axon Initial Segment in Cortical Principal Neurons Is Caused by the Na-K-2Cl Cotransporter NKCC1 Journal of Neuroscience, 28 (18), 4635-4639 DOI: 10.1523/JNEUROSCI.0908-08.2008
Molnár, G., Oláh, S., Komlósi, G., Füle, M., Szabadics, J., Varga, C., Barzó, P., & Tamás, G. (2008). Complex Events Initiated by Individual Spikes in the Human Cerebral Cortex PLoS Biology, 6 (9) DOI: 10.1371/journal.pbio.0060222
Appendix: You can use the back key to return to where you were, or click on the quoted text to return to the line with the footnote.
A1. "axon initial segment" There's no link to Wiki because, as of this writing, I couldn't find anything on Wiki discussing it. So herewith...
As mentioned above, the AIS is the first part of the axon, "from the narrow beginning of the axon to the onset of the myelin sheath" (often more than 200 microns (μ))[35], or in unmyelinated axons "a variable distance, 4-5 μ." It has recognizable features:[31]
The initial segment of the axon is unlike any other known process of the nerve cell, and in certain respects it is unlike any other part of the axon itself. In the idealized nerve cell it arises from the summit of a conical projection on the surface of the perikaryon, the axon hillock (Fig. 1). The surface of the axon hillock is bounded by a plasma membrane with the usual trilaminate structure, but near the apex of the hillock (Fig. 2) a thin, dark layer of finely granular material appears just beneath the membrane. In low-power electron micrographs this granular material gives the membrane a dense appearance, so that on superficial examination the plasmalemma seems to be reduplicated or thickened. But the undercoating is not really a part of the limiting membrane; instead, it is a thin layer of powdery densities, about 100 Å [Ångströms] thick, separated from the surface membrane by a clear interval of about 30 Å (Figs. 3 and 5). As its margins are not distinct, these measurements cannot be precise. The undercoating extends from the narrow beginning of the axon to the onset of the myelin sheath, where it ceases as abruptly as it began. In unmyelinated axons the undercoating extends for a variable distance, 4-5 μ. Where the axon originates from a dendrite, the undercoating has the same sudden onset and is accompanied by other changes (see below) in the internal structure of the process that signal the beginning of an axon.[31]The above text, and the figures it references, are in Reference 31, which is open access, although it's rather old.
Both the axon hillock and the AIS are distinct compartments within the cell, which can be independently targeted by distinct mixes of specific receptors, ion pumps, and all the other types of machinery the cell uses.[27] [28] [29] Ultrastructurally:
In the axon hillock (Fig. 2) microtubules collect into bundles of three to five or more, which funnel into the initial segment and run parallel with one another throughout its length. Large initial segments have five or six such fascicles, while small ones may have only one. The number of microtubules in a bundle varies considerably but is probably characteristic of the type of neuron. In the Deiters cells of the lateral vestibular nucleus (Fig. 5) and in the motor neurons of the spinal cord, the number of microtubules included in a fascicle is usually three to five, and in the pyramidal cells of the cerebral cortex (Fig. 6) the number can reach 22. In the Purkinje cell of the frog, Kohno (13) found from 6 to 25 microtubules in a bundle, but in the rat the maximumThese "synaptic boutons attached to the surface of the perikaryon at the axon hillock" and "the initial segment of the cerebral pyramidal cell" are at the center of the primary thrust of this post.
number we have found in this type of neuron is only 10-12.In longitudinal sections the fasciculated microtubules in the initial segment often appear darker than the single microtubules in the rest of the nerve cell and its processes. This appearance is due only partly to overlapping of the microtubules in a bundle within the thickness of the section. In addition, each microtubule is surrounded by a cloud of fine fibrillar material that contributes to the general density of the fascicle. In transverse sections it can be seen that the microtubules are arrayed close together in a curving and sometimes branching line (Figs. 3, 4, and 6). Single or isolated microtubules are rarely encountered in the initial segment. Favorably oriented transverse sections show that the microtubules within the fascicles are bound together by thin, dark crossbars or arms (Figs. 3 and 6).
The bundling of the microtubules ceases abruptly at the beginning of the myelin sheath. Whether they continue down the axons as isolated microtubules or are replaced by new tubules beginning in this region could not be determined from the sections that we examined.
Although the axon hillock and the beginning of the axon fail to stain with basic dyes, clusters and rosettes of ribosomes do occur in the axon hillock and, in diminishing quantities, throughout the length of the initial segment. Apparently they are not numerous or concentrated enough to produce a basophilia that is recognizable in the light microscope. The ribosomes are usually, but not always, associated with a tubule or two of the endoplasmic reticulum. At the beginning of the myelin sheath they disappear while the endoplasmic reticulum continues in its agranular form throughout the axon.
Other cytoplasmic components of the axon, the neurofilaments, the mitochondria, multivesicular bodies, and various vesicles, all pass into the axon from the axon hillock without undergoing any distinctive change in their appearance or aggregation. Near the apex of the axon hillock the mitochondria, neurofilaments, microtubules, and endoplasmic reticulum all assume a remarkably parallel orientation as they funnel into the narrow initial segment.
It is common to find synaptic boutons attached to the surface of the perikaryon at the axon hillock, but they are unusual on the surface of most initial segments. For example, in sections through the initial segments of some 60 different Purkinje cells only one synapsing bouton was found. In contrast, nearly every section through the initial segment of the cerebral pyramidal cell shows an attached bouton (Fig. 7). No examples have yet been encountered of initial segments studded with boutons like the dendrites and perikaryon of certain cells.
In a few cases in which the apposition between an axon terminal and the initial segment was caught in a favorable plane of section, it was possible to see that the typical undercoating of finely granular material was interrupted at such sites and that the surface of the axon reverted to the appearance it normally has in the internodal segments. Only at the location of the "synaptic complex" or "active zone" was there a deviation from the normal, here resulting from the aggregation of fine filamentous material that formed the postsynaptic density. The typical undercoating of the initial segment resumed beyond the margin of the apposing terminal. If a neuroglial process was inserted between the terminal and the axon, breaking the apposition, the typical undercoating reappeared beneath it. In our material the number of cases in which we could clearly follow both the pre- and postsynaptic membranes was too small to allow us to generalize this description with assurance.[31]
The AIS is the most common site for an action potential to begin, followed by the axon hillock. As the general dendritic excitement (depolarization) increases, the site often moves back, first to the soma, then sometimes to the proximal dendrite(s).[35]
Links: (I've only included the links called out in this leader.) Not all of these are called out in the text. Use the back key if you came via clicking a footnote above.
1. Excitatory Effect of GABAergic Axo-Axonic Cells in Cortical Microcircuits requires free registration
2. Cation–chloride co-transporters in neuronal communication, development and trauma paywall
3. Chloride is preferentially accumulated in a subpopulation of dendrites and periglomerular cells of the main olfactory bulb in adult rats paywall
4. Cluster Analysis–Based Physiological Classification and Morphological Properties of Inhibitory Neurons in Layers 2–3 of Monkey Dorsolateral Prefrontal Cortex Open Access
5. Output of neurogliaform cells to various neuron types in the human and rat cerebral cortex Open Access
6. A Population of Prenatally Generated Cells in the Rat Paleocortex Maintains an Immature Neuronal Phenotype into Adulthood paywall Is this one relevant?
7. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system paywall
8. A subset of periglomerular neurons in the rat accessory olfactory bulb may be excited by GABA through a Na+-dependent mechanism paywall
9. Channel behavior in a gamma-aminobutyrate transporter Open Access
10. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance Open Access
11. Structure of intraglomerular dendritic tufts of mitral cells and their contacts with olfactory nerve terminals and calbindin-immunoreactive type 2 periglomerular neurons paywall
12. Compensatory changes in cellular excitability, not synaptic scaling, contribute to homeostatic recovery of embryonic network activity paywall
13. Enigmatic Central Canal Contacting Cells: Immature Neurons in "Standby Mode"? paywall
14. Combined Analog and Action Potential Coding in Hippocampal Mossy Fibers Requires free registration
15. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential
16. Long-Term Activity-Dependent Plasticity of Action Potential Propagation Delay and Amplitude in Cortical Networks Open Access
17. Information processing by graded-potential transmission through tonically active synapses
18. Enhancement of presynaptic neuronal excitability by correlated presynaptic and post-synaptic spiking
19. Recurrent excitation in neocortical circuits paywall
20. GABAergic Depolarization of the Axon Initial Segment in Cortical Principal Neurons Is Caused by the Na–K–2Cl Cotransporter NKCC1 Open Access
21. Complex Events Initiated by Individual Spikes in the Human Cerebral Cortex Open Access
22. Axonal GABAA receptors paywall
23. Robust Short-Latency Perisomatic Inhibition onto Neocortical Pyramidal Cells Detected by Laser-Scanning Photostimulation paywall
24. Excitatory GABAergic Activation of Cortical Dividing Glial Cells paywall
25. GABA Transporter GAT1 Prevents Spillover at Proximal and Distal GABA Synapses Onto Primate Prefrontal Cortex Neurons paywall
26. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations
27. Compartmentalizing the neuronal plasma membrane from axon initial segments to synapses paywall
28. Localization and Targeting of Voltage-Dependent Ion Channels in Mammalian Central Neurons paywall
29. The distribution and targeting of neuronal voltage-gated ion channels
30. Excitatory effects of GABA in established brain networks paywall
31. The axon hillock and the initial segment Open Access
32. Proximity of excitatory and inhibitory axon terminals adjacent to pyramidal cell bodies provides a putative basis for nonsynaptic interactions paywall
33. The cellular, molecular and ionic basis of GABAA receptor signalling
34. Salient features of synaptic organisation in the cerebral cortex
35. From molecules to networks : an introduction to cellular and molecular neuroscience edited by John H. Byrne, James Lewis Roberts, chapter 17

Hi all,
ReplyDeleteWhen the intracellular chloride concentration was 50mM and the internal concentration of adenosine 5'-triphosphate (ATP) was 4mM, the average chloride influx was 11.6 pmoles/cm2 x sec. When the axons were dialyzed with an ATP-free solution, the average influx fell to 5.1 pmoles/cm2 x sec. The effect was fully reversible upon the return of ATP to the dialysis fluid. Thanks for sharing this information.